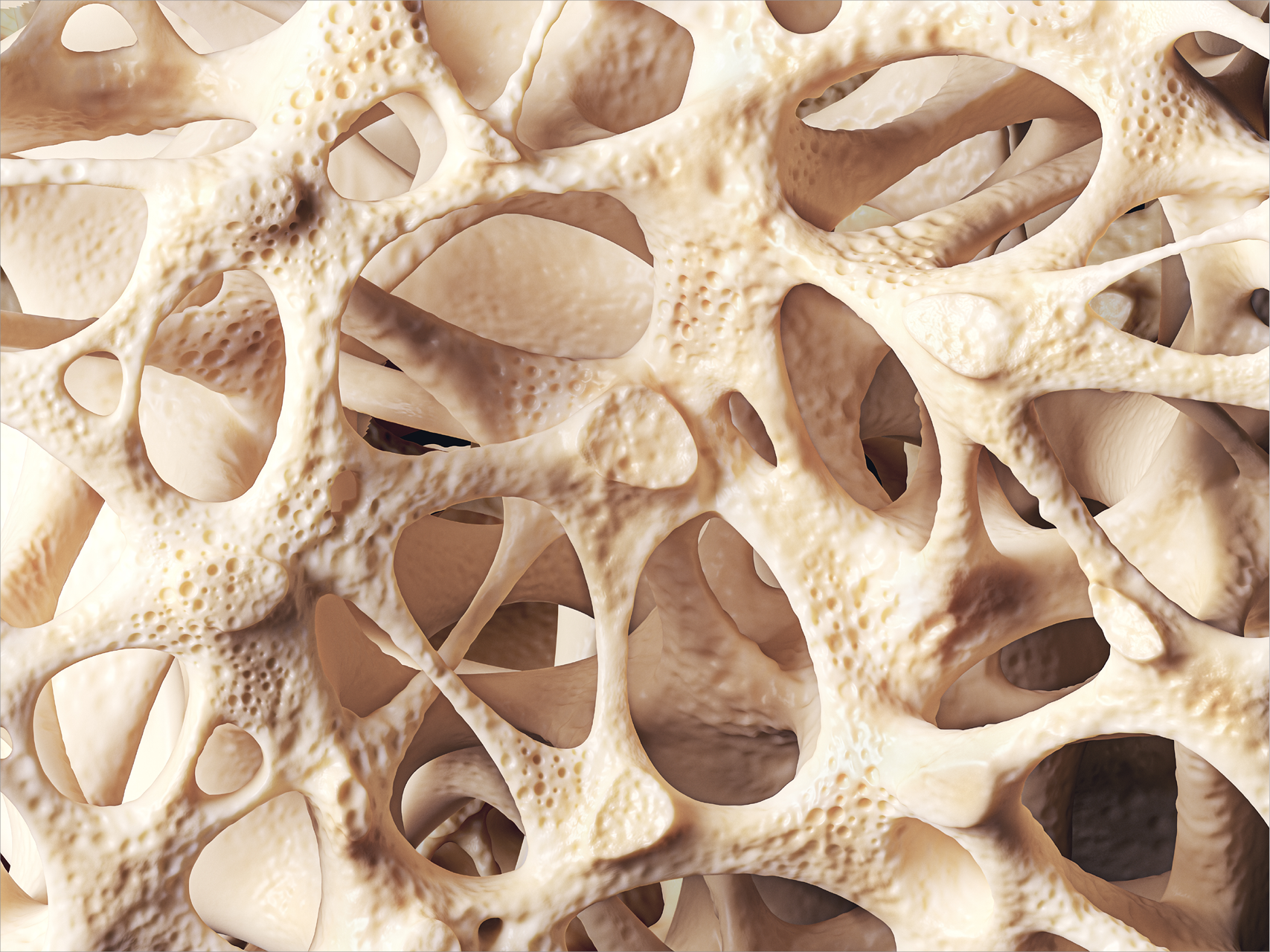
The Osteograft product world
In Germany, allogeneic cell and tissue transplants are regulated and subject to approval as medicinal products, which means that their production, biological safety and clinical use are subject to constant monitoring by the German authorities. The DIZG has 10 marketing authorizations for human tissue transplants.
Allogeneic transplants
The system for ensuring the biological safety of DIZG tissue transplants consists of extensive criteria for donor selection, comprehensive serological donor screening that far exceeds the requirements of EU Directive 23/2004 and includes 4 virus genome examinations, the use of a validated procedure for the removal/inactivation of viruses, bacteria and fungi, and in-process and final checks on the transplant.
The DIZG and ARGON are certified according to DIN EN ISO 13485 and follow the Ethical Code and the quality standards of the European Association of Tissue Banks (EATB).
The use of DIZG transplants is subject to documentation in accordance with the Transplantation Act (TPG).
For this purpose, labels for the patient file are enclosed with each package.
All dimensions are approximate and minimum values. They are subject to variation depending on the preparation and tissue. Explanations and instructions for the application of the transplants can be found in the instructions for use and technical information enclosed with each package.

The non-profit DIZG - German Institute for Cell and Tissue Replacement

Applications/indication
Quickfinder products:
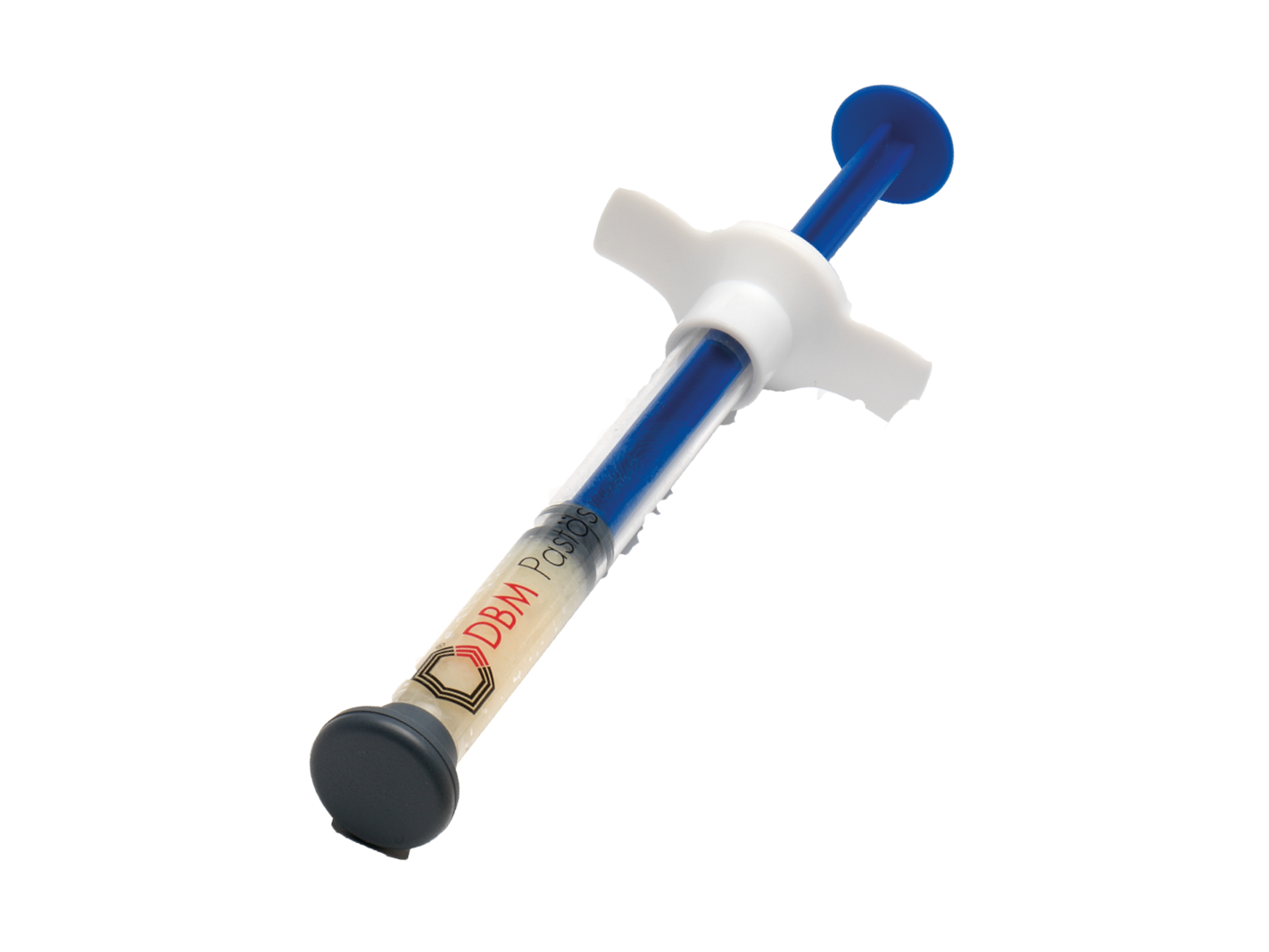
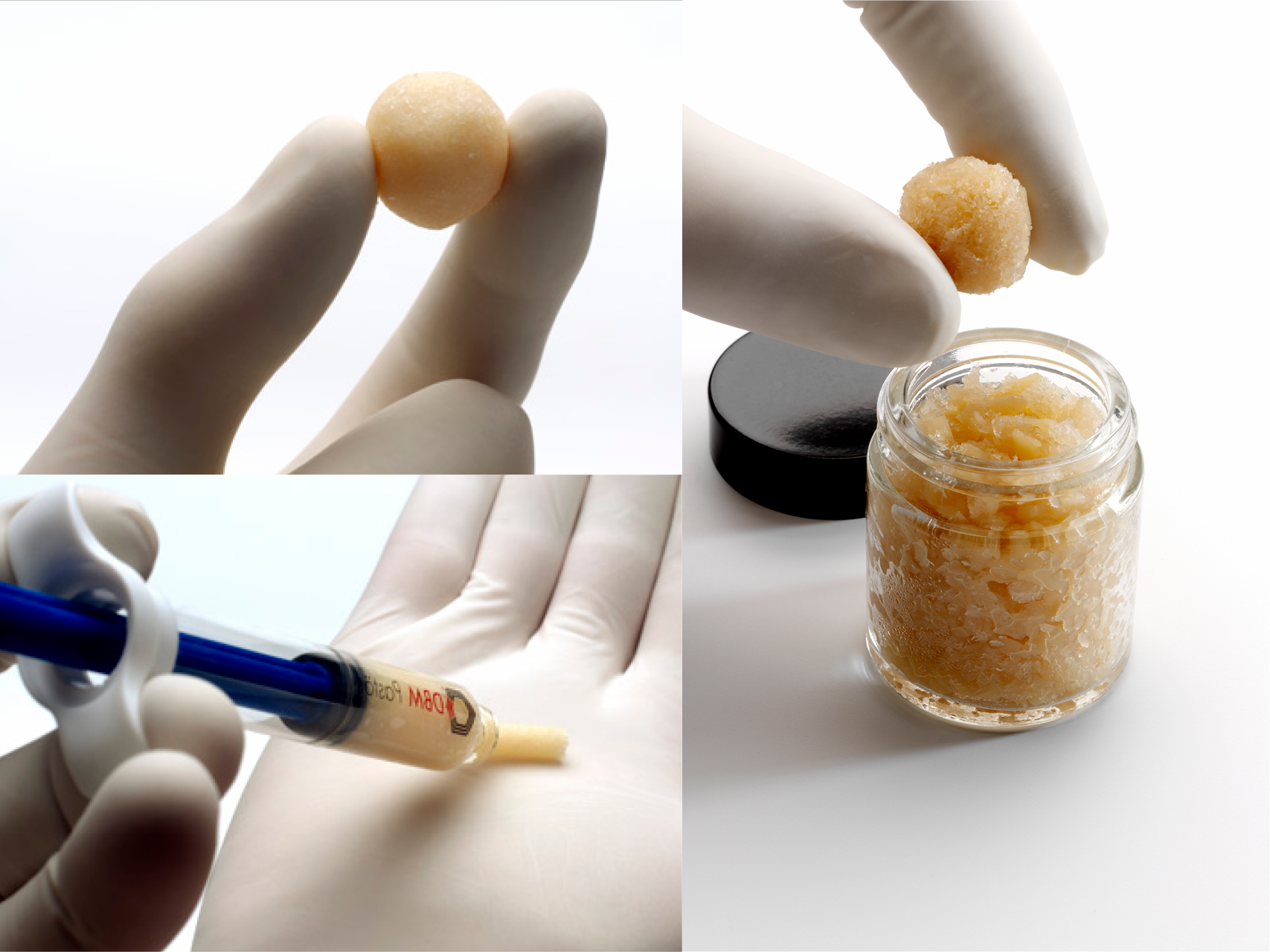
Demineralized bone matrix (DBM) pasty
Demineralized bone matrix (DBM) in paste form is a pioneering product for augmentation. It promotes bone growth and healing, improves bone quality and optimizes surgical results.
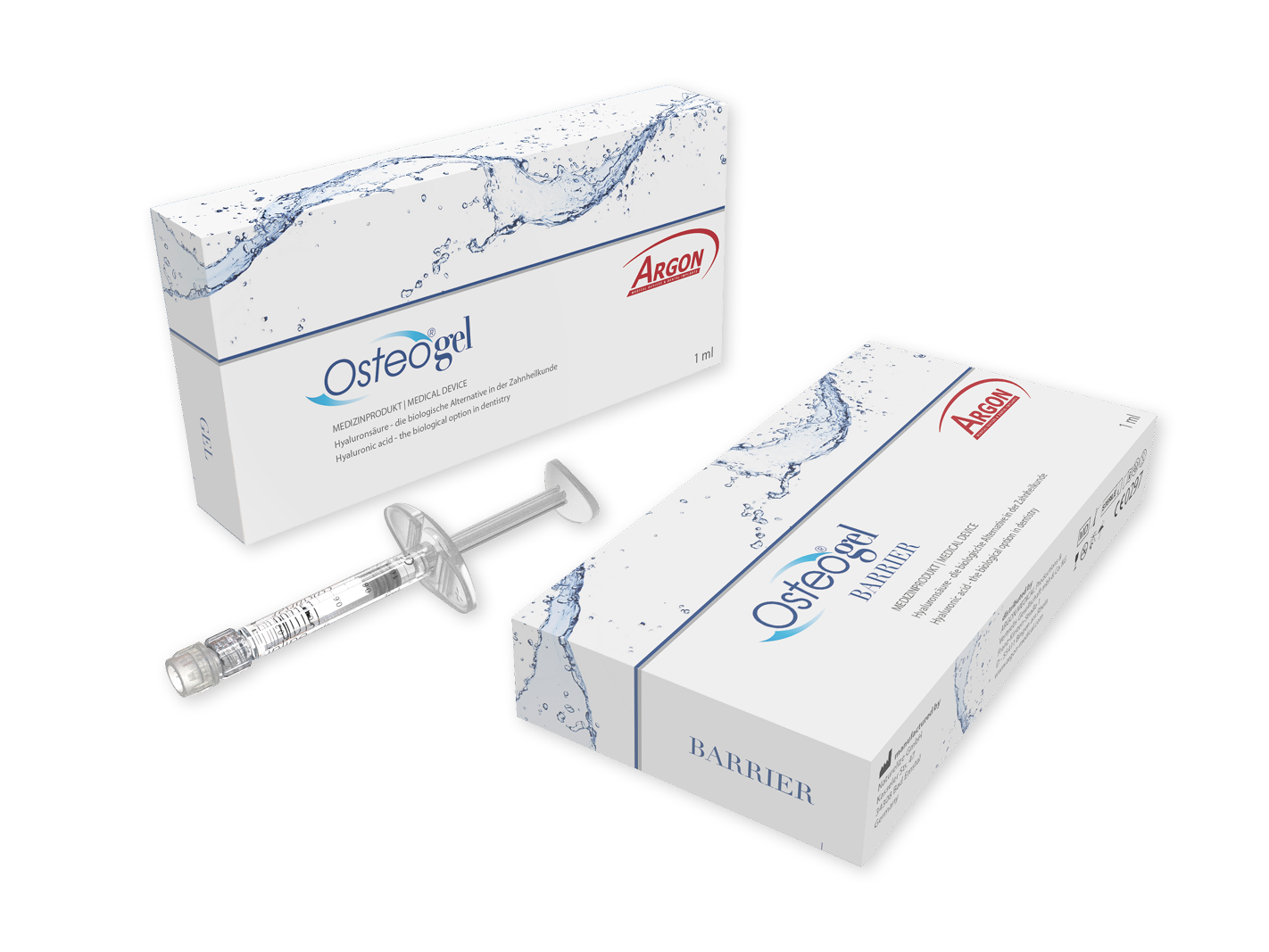
Osteogel
High-precision, modular systems that can be customized according to your requirements and the thread designs and lengths you use, but in one place!